THE PROBLEM
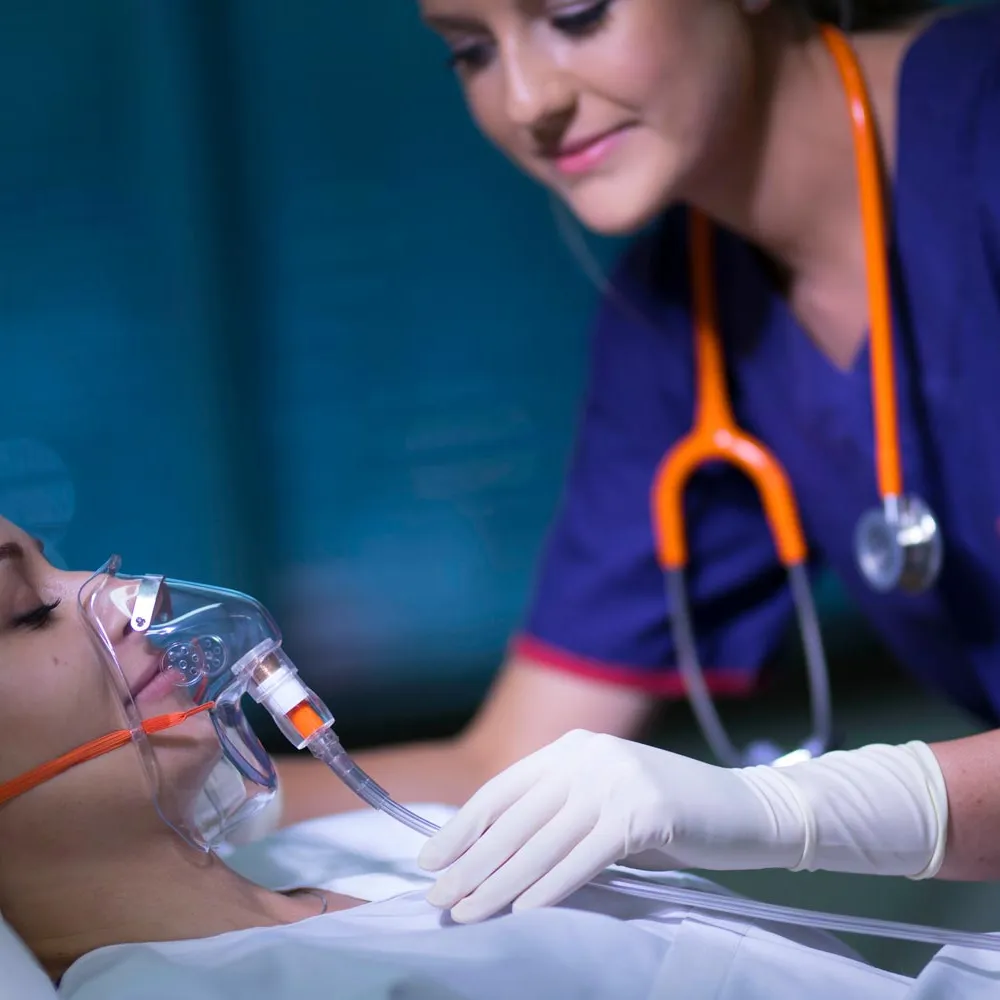
Leading Melbourne anaesthetist, Dr Matthew Matusik, identified a potential risk for patients undergoing surgical procedures requiring supplementary oxygen therapy – especially frail or at-risk patients. Dr Matusik had studied the well documented global cases of patients in theatre suffering from hypoxemia (low blood oxygen) due to an oxygen supply blockage. Using his many years of experience at St Vincent’s Hospital, he was aware that this serious issue could be due to many causes. But the main ones were equipment failure (i.e. a kink in the oxygen hose), human error (i.e. accidental disconnection), or an unattended (empty) oxygen bottle. The consequences are severe resulting in brain injury or even death.
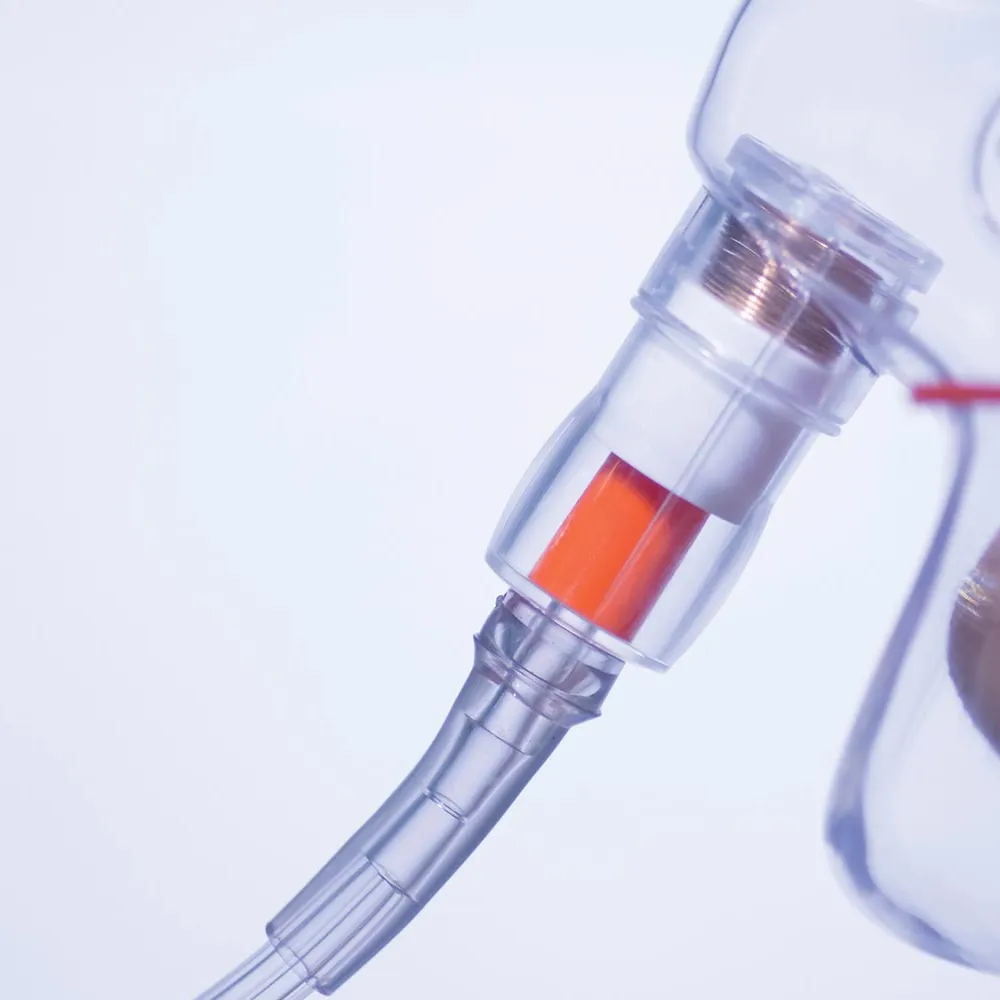
Finding a solution was the first problem for Dr Matusik in inventing a safety management valve called the SureFlO2. But finding a suitable manufacturer to produce medical procedure components became an even bigger issue for the doctor.
Enter Forme Technologies. They became the ideal fit due to:
- Transforming an idea into a robust working solution for the medical sector
- Offering a manufacturing platform and solution suitable for mass production of medically rated equipment
- Working within all compliance guidelines and regulatory bodies in order to take it to market.
THE BRIEF
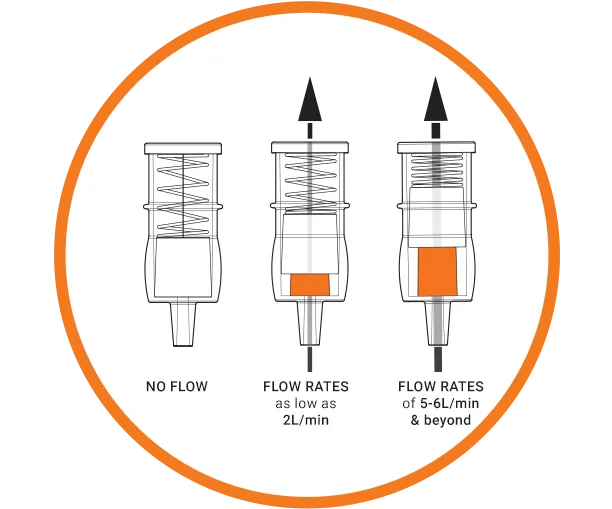
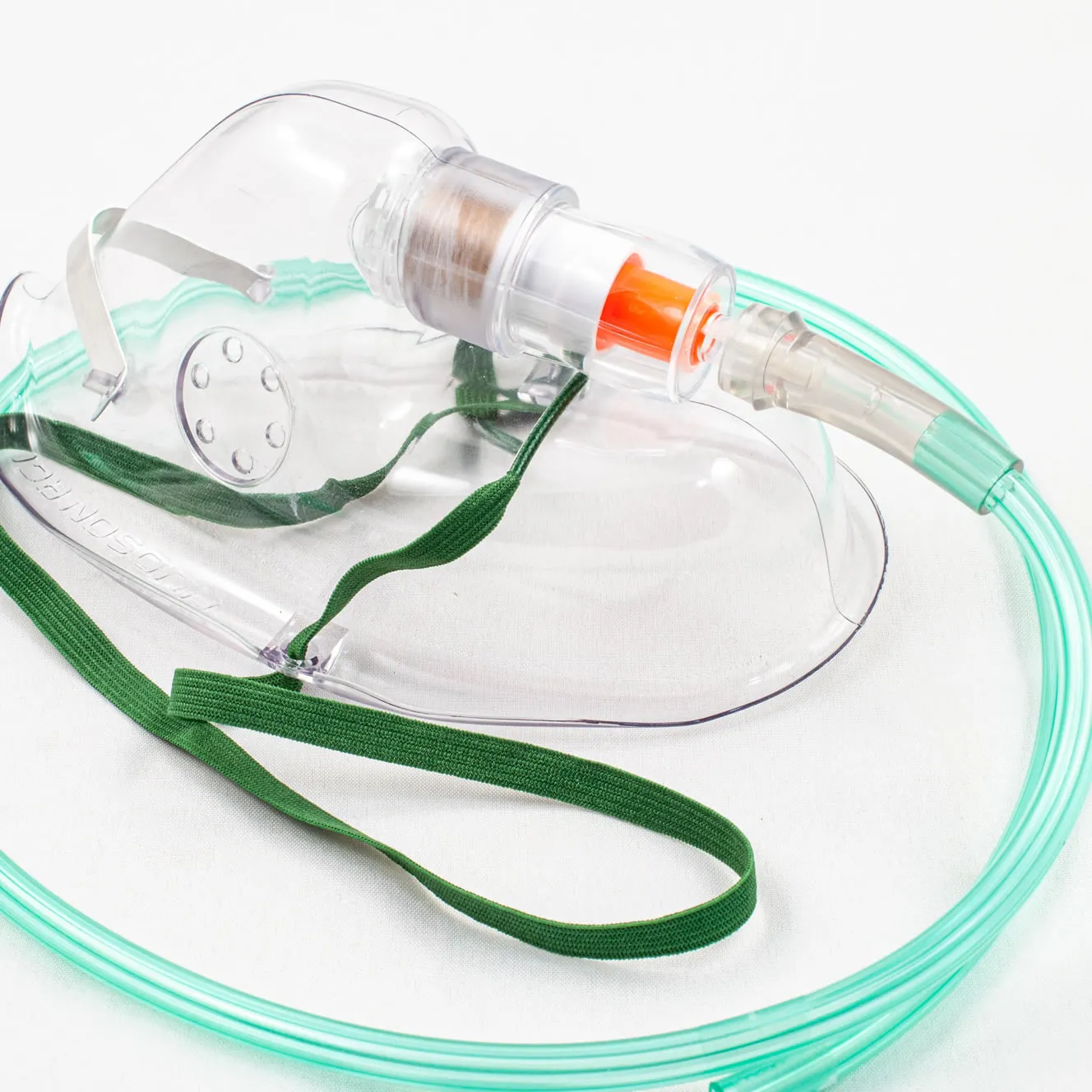
Take Dr Matusik’s idea and turn it into a fully functioning medical device from the design stage, the development stage, right through to manufacturing. This device has to be able to show that the oxygen supply is working when delivering oxygen to the patient’s oxygen mask.
The following issues also had to addressed in the design process:
- The device must function to specification regardless of a patient’s position.
- The indication of flow should be immediately clear and visible – even from a distance.
- The device must be fail-safe.
- The device must be MRI transparent.
- The device has to be manufactured to ISO 13485 guidelines.
- The materials used for its construction must be biocompatible and compliant with applicable 10993 and 18563 standards.
- The device must be Australian Regulatory Body compliant and bearer of the CE Mark.