THE PROBLEM
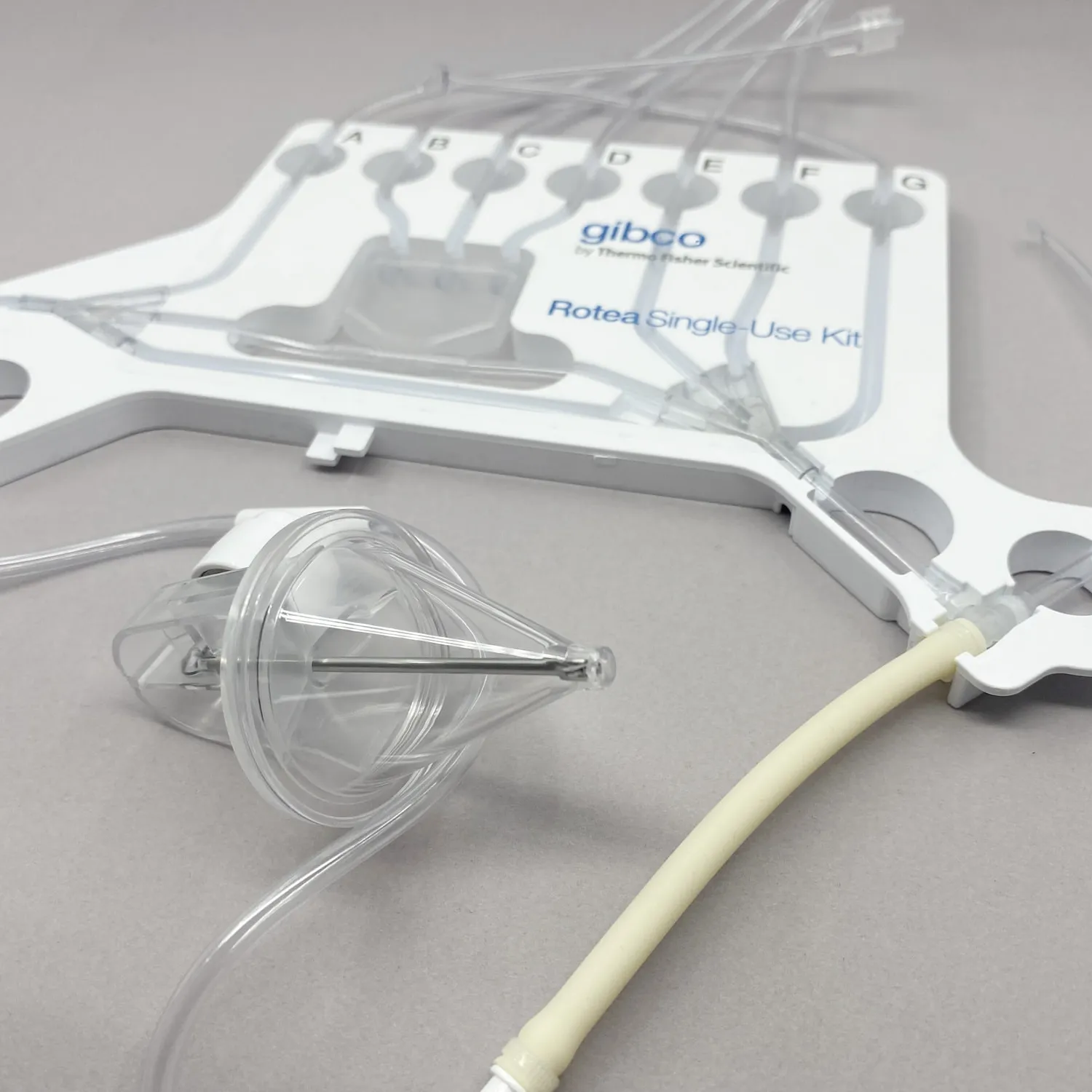
The team that invented and developed Rotea approached Forme Technologies with a functional prototype of their impressive technology. They had passed the research and development stage and needed to transfer the device to manufacturing. Prototype components were not sufficient anymore. They were in the hunt of a manufacturing partner that could fabricate its product to stringent technical specifications and under the guidelines of ISO 13485.
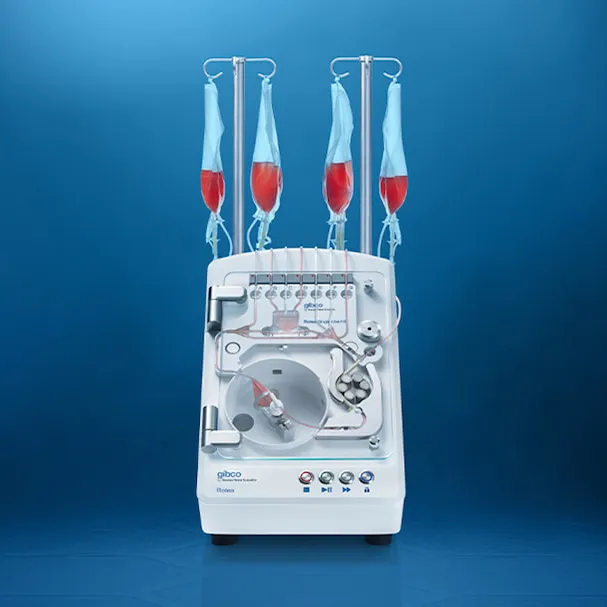
The technology of Rotea is based on the principle of counterflow centrifugation where high rotational speeds of up to 8000 rpm are reached to process the cells. The prototype parts used to concept prove Rotea were not dimensionally consistent. In isolated cases they were generating excessive runout that translated in vibration resulting in separation of the parts and breakage. In addition to this, the dimensional variation increased the complexity in the assembly line and scrap was generated.
- Touch free manufacturing.
- Tight tolerances +/- 0.05mm
- Developed under the guidelines of ISO 13485.
- Design for manufacturability
THE BRIEF
Tool development and manufacturing of single-use components for a cell therapy device in Polyethylene terephthalate glycol - PETG with minimal or no human contact.
- Biocompatible polymer.
- Clean environment manufacturing.
- Steel like tolerances.
- Manufactured under ISO 13485 guidelines.
- Must be able to assist product validation and generate supporting documentation.