THE PROBLEM
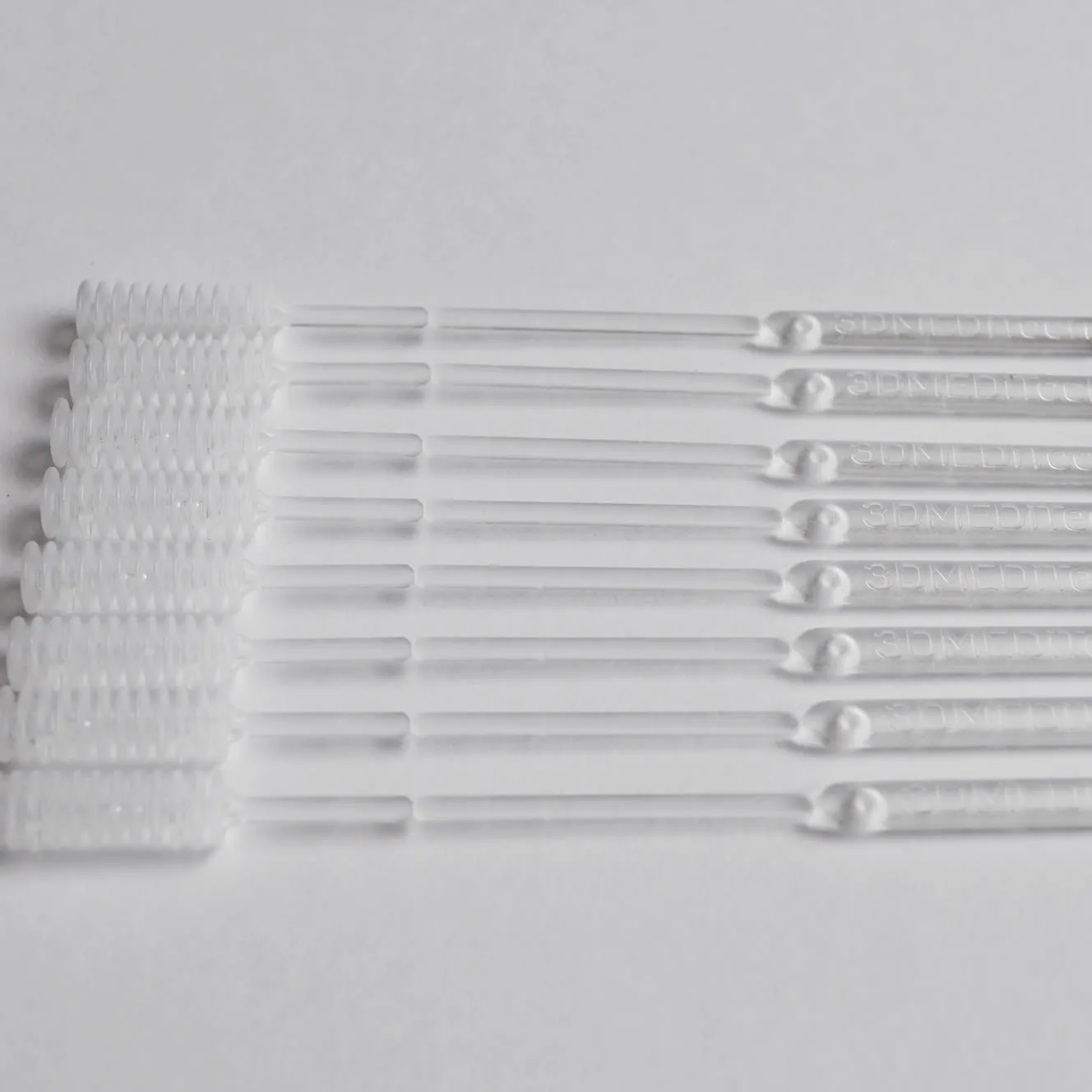
3dMeditech developed nasopharyngeal testing kits and saliva swabs to fight the COVID-19 virus. The swap used to take the saliva sample is manufactured by 3dMeditech using high end 3d printers. They were pioneers in the world in the use of 3d printers for swab manufacturing. With an increased demand the production throughput was not enough and as a result 3dMediTech needed to increase their production output in a short time. They identified the injection moulding process as the solution.
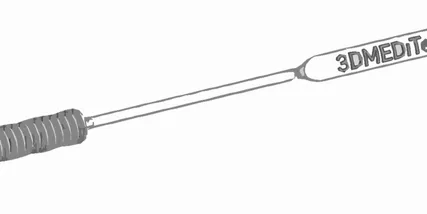
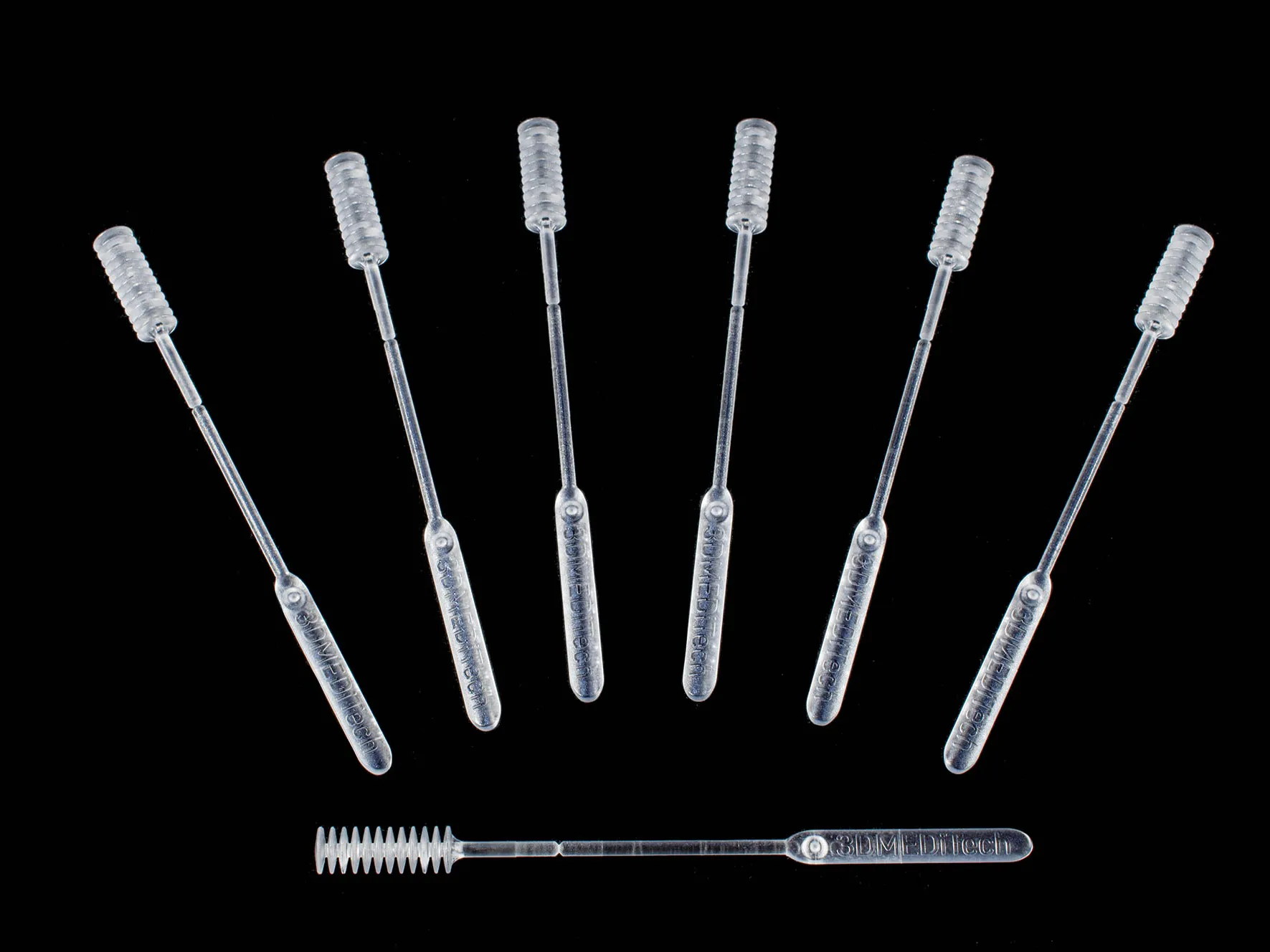
The potential injection moulded swab should retain the two main features of the 3d printed swab. 3dMeditech incorporated and innovative feature to capture the adequate volume of saliva for the test. The handle of the swab can be break off from the sample shaft so it can be stored in the sample container for preservation and transportation.
That is when 3dMediTech approached Forme Technologies.
They were looking for a partner that could reengineer their 3dprinted swab for injection moulding and be production ready within 3 weeks.
- Design optimisation for injection moulding.
- Short lead time for tool manufacturing
THE BRIEF
Optimise 3dMeditech’s swab design for injection moulding to increase production capacity without compromising it’s performance.
- Modify the design to be produced injection moulding.
- Identify a biocompatible material that meets the requirements of the 10993 standard.
- The mechanical characteristics of the new material must meet the ones of the 3d printed material at a similar cost.
- Separate the swab from the handle, the swab breaking feature must perform as the current 3d printed part.
- The solution should be ready in less than three weeks.
- The swab must be produced in a clean environment without human contact.
![DSC_0115[1]](https://formetechnologies.com.au/image/gocx4wQ-lM0BLs0GSAKTAQEA/DSC_0115-1.webp)