

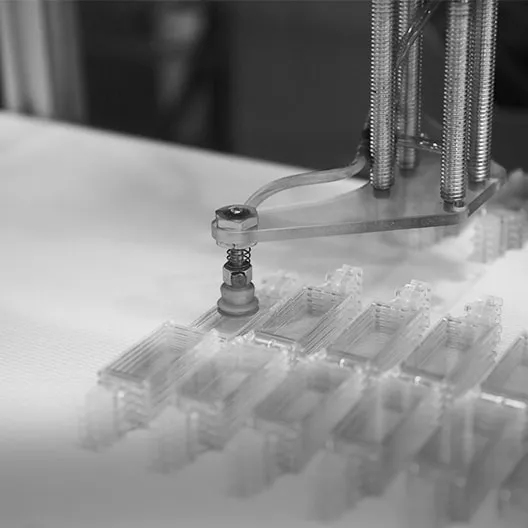




Forme Technologies is one of the few companies in Australia that have injection moulding and value adding capabilities inside an ISO 7 Cleanroom.
Thanks to our new cleanroom we can guarantee that the cleanliness specifications of critical components as per the ones demanded by the medical devices, and bio-technology industries are met.

The facilities where our ISO 7 cleanroom is housed are ISO 13485 certified. This means that your medical device or critical component is being manufactured to the highest standards of quality and cleanliness.
Our personnel are highly trained and experienced which allow us to run a quality-conscious production.
Forme Technologies can help you to navigate the regulatory complexities of the medical devices industry and ease your path to market.

Inside our cleanroom we also perform downstream operations and assembly using automated (robots or bespoke machines), manual and/or hybrid solutions.
We also have clients outside the medical field that benefit from our cleanroom and the services we provide.

Additionally, we have an in-depth knowledge of environmental requirements such as recyclability, flammability, and UV sensitivity, ensuring that your product meets or exceeds, all required environmental regulations.




